During World War I, Germany was able to sustain the war despite blockades and resource shortages. At the heart of this was a remarkable catalytic technology called the Haber-Bosch process, which synthesized ammonia to produce fertilizers and explosives. This technology revolutionized Germany’s agricultural production and munitions industry, and was crucial to keeping the war going.
World War I began on July 28, 1914, with Austria’s declaration of war on Serbia, and ended on November 11, 1918, with Germany’s surrender. The war was fought between the German-Austrian alliance and the Allied powers of Great Britain, France, and Russia. Japan and the United States also joined the war, which would affect the entire world. Initially, Germany expected a short war, but when the allies lost early on, the war turned into a long war. As it became a protracted war, securing and supplying supplies became a major issue. Let’s focus on Germany. What drove Germany to continue the war despite its early setbacks? Germany’s drive to wage war is closely tied to its “catalysts.
Let’s take a quick look at catalysis. Catalysis was first conceptualized by the Swedish chemist Vergelius. He discovered catalysis in a mineral called zeolite. As defined by the International Union of Pure and Applied Chemistry (IUPAC), a catalyst is a substance that increases the rate of a reaction but is not consumed during the reaction. How does it increase the rate of a reaction? Because the catalyst changes the reaction pathway and lowers the activation energy. You can think of it similarly to climbing a mountain, but instead of climbing it directly, you take another, easier route through a tunnel. As you can see in the figure below, the energy barrier that needs to be overcome is lowered by the catalyst.
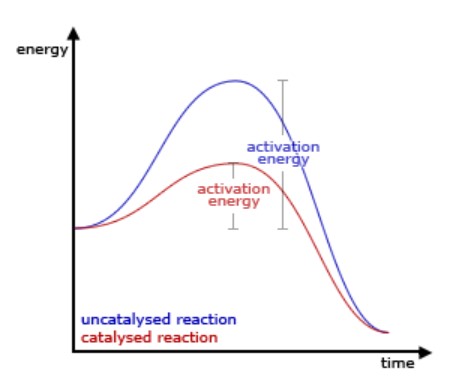
(Source – https://ch302.cm.utexas.edu/kinetics/catalysts/catalysts-all.php)
The catalyst is closely related to Germany’s war effort. Germany was a powerhouse in the field of chemical engineering around 1900. Of course, it is still a powerhouse today, but during the First World War, it had the status of a superpower and the level of catalyst technology was probably quite high. By utilizing their catalyst technology, Germany was able to start the war well prepared. Until 1913, Germany used Chilean chalk (sodium nitrate and potassium nitrate) as a raw material to make gunpowder. The problem with this raw material was that it had to be transported from South America to Europe, and there was never enough of it in absolute quantities. However, in 1913, two chemists named Haber and Bosch developed a process to synthesize ammonia (NH3) from readily available materials. An iron (Fe) catalyst is used, and the chemical formula is as follows

The resulting ammonia is converted to nitric acid, which is then processed and used as TNT. TNT stands for TriNitroToluene and is the raw material for dynamite. Ammonia was not only used for explosives, but also for food production. Ammonia was converted into urea, which was used as a fertilizer. Naturally, food production increased. We can see that catalyst technology contributed greatly to two aspects: weapons and food.
Now let’s look at another aspect. Germany is very rich in coal. In order to ensure enough oil, Germany devised a way to make synthetic petroleum from coal. Bergius and Fischer developed their own methods: first, Bergius liquefied coal directly to make synthetic petroleum. Fischer devised a more complex method. First, the coal is converted into syn gas (synthesis gas, composed mainly of carbon monoxide and hydrogen), which is then converted into synthetic petroleum under iron (Fe) and cobalt (Co) catalysts.
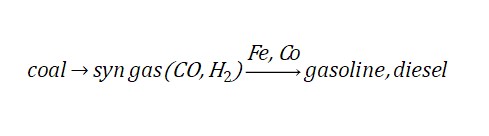
The process of converting syn gas to oil is called the Fischer-Tropsch process. The amount of synthetic petroleum produced accounted for 95% of aircraft fuel in World War II and nearly 60% of Germany’s total oil supply. The Allies even capitalized on this fact when they attacked Germany in the final months of the war. In 1944, the Allies bombed Germany’s synthetic petroleum plants, and the fuel shortage led to Germany’s defeat. Here we see that catalyst technology made an important contribution to the “fuel” side of the equation.
Germany’s superior catalyst technology was one of the reasons why it was able to fight the First World War. The abundance of coal also had a positive impact. Specifically, ammonia synthesis increased the production of fertilizers and dynamite, while synthetic petroleum production solved the fuel problem. The discussion so far has shown that catalytic technology is linked to the critical elements of warfare: food, weapons, and fuel.