Hydrogen storage alloys are a technology that stores hydrogen densely within metals to increase energy density and address stability and cost issues. This is expected to accelerate the commercialization of hydrogen energy.
Hydrogen is the smallest and lightest substance that exists in a stable state in the universe. But its power is greater and more far-reaching than any other substance. Hydrogen is an energy source with endless potential for development and is a pillar of renewable energy, also known as the energy of the future. It’s already being used as a fuel for spacecraft and fuel cells, and it’s expected to become an increasingly important source of energy in the future. However, hydrogen is not without its drawbacks, and there are still many steps to be taken before it can be commercialized. The main disadvantage of hydrogen is its energy density. Energy density is a measure of how much energy a liter of a fuel can produce. Since hydrogen exists as a gas at room temperature, it takes up a lot of volume, which means that to get a certain level of energy from it, you need a huge storage container. If hydrogen were a solid metal, such as iron, it would be possible to get a lot of energy from a small volume. This is where the idea of storing hydrogen densely inside a metal comes from.
Although hydrogen is not a real metal, if it is stored at a high density in a metal, it can be stored conveniently and generate a lot of energy as if it were a metal. In other words, the energy density of hydrogen can be greatly increased depending on the storage method, which is a step towards the commercialization of hydrogen energy. These alloys are called “hydrogen storage alloys” because they are made by mixing special metals to store hydrogen inside them and take it out again when needed. This hydrogen storage technology is essential for commercializing hydrogen energy. But how do hydrogen storage alloys work, what are the different types, and what are the current advantages and technical limitations of this technology?
According to the chemist Dolton, the world is made up of particles called atoms, which means that it is not a continuous substance, but a collection of discrete particles. Metals are also made up of metallic bonds between metal atoms. Since metals are not a continuous substance, there are spaces between metal atoms, and these spaces can be used to store hydrogen. If the properties of the metal can be modified to store hydrogen in the spaces between the atoms and take it out again when needed, it can be used as a hydrogen storage alloy. Metals combine with hydrogen to create a substance called a metal hydride. For example, magnesium combines with hydrogen to form magnesium hydride (MgH2). For some metals, the metal hydride formed by hydrogenation is more stable than the original metal, while for others, the metal hydride is more unstable than the original metal. For example, metals such as magnesium and lanthanum have a strong affinity for hydrogen, so they have a strong tendency to combine with hydrogen. On the other hand, metals such as iron and nickel do not like hydrogen and are strongly inclined to break the bond with hydrogen.
Let’s look at the behavior of alloys that mix these two different metals. When hydrogen is added to this alloy, the hydrogen-loving metal atoms will interact with the hydrogen in such a way that they capture it into the metal for bonding. On the other hand, metal atoms that are not friendly to hydrogen will interact in the direction of exporting hydrogen out of the metal because they become unstable when combined with hydrogen. Therefore, if the interaction between hydrogen and friendly metals is large, hydrogen will be stored inside the metal, and if the interaction between hydrogen and unfriendly metals is large, the hydrogen stored inside will be released outside the metal. The magnitude of the interaction between the metal and hydrogen depends on the external conditions such as temperature, pressure, and electrical force. Therefore, by adjusting the thermodynamic and electromagnetic conditions, hydrogen can be stored inside the metal and then released when needed by adjusting the external conditions again. These alloys are called hydrogen ‘storage’ alloys because they act like a storage container that releases fuel when a valve is opened.
So, what are the different types of hydrogen storage alloys? Hydrogen storage alloys can be categorized into two types based on how they control external factors. There are those that control the interaction between the metal and hydrogen by controlling thermodynamic factors such as pressure and temperature, and those that control electromagnetic factors such as voltage and current. First, let’s look at hydrogen storage alloys that store hydrogen by controlling thermodynamic factors. This hydrogen storage alloy uses the following reaction equation
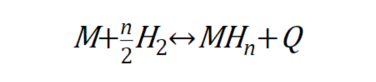
This equation means that the alloy M combines with gaseous hydrogen to form metal hydrides (MH_n) and release heat. If you increase the pressure and decrease the temperature of this alloy, the interaction of hydrogen with the neighboring metal atoms will be stronger and hydrogen will be stored in the alloy. On the other hand, lowering the pressure and raising the temperature strengthens the interaction with metal atoms that are not friendly to hydrogen, and hydrogen is released outside the alloy. Therefore, it is a kind of hydrogen storage alloy that can store hydrogen in the gaseous state under low temperature and high pressure, and when hydrogen is needed, it can be drawn out by lowering the pressure and raising the temperature. On the other hand, hydrogen storage alloys that store hydrogen by controlling electromagnetic factors use water as a source of hydrogen rather than gaseous hydrogen. Water is composed of hydrogen and oxygen, and electrical forces can be used to remove hydrogen, a component of water molecules, and store it in the alloy. An electrolyzed hydrogen storage alloy uses the following reaction formula
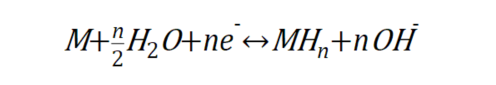
This is an electrochemical reaction between alloy M and water in which hydrogen is stored in the alloy and hydroxide ions are produced. If the alloy is placed in water and connected to a low-potential cathode (e.g., the (-) pole of a battery), the interaction between the hydrogen-loving metal and the hydrogen is strengthened, and the reaction of storing hydrogen by supplying electrons occurs. On the other hand, when connected to a high-potential anode, the reaction is the opposite, releasing electrons and releasing hydrogen. Thus, hydrogen is stored in the metal while the potential is low, and when hydrogen is needed, the potential can be increased to draw hydrogen out of the metal and use it as fuel.
So what are the advantages of this hydrogen storage technology over other hydrogen storage methods? The other leading technology for storing hydrogen at high energy density is liquid hydrogen storage. It is true that liquefying hydrogen and storing it as a liquid reduces its volume dramatically, so it can be stored at a high energy density. However, the temperature at which hydrogen begins to exist as a liquid is minus 253 degrees Celsius, and maintaining cryogenic temperatures is extremely expensive. Also, because hydrogen exists as a pure hydrogen molecule without any mechanism, there is a huge risk that even a small spark can cause a large explosion. However, hydrogen storage alloys can solve both of these existing problems. Of course, it is necessary to change the external environment to store hydrogen, but this can be done at a fraction of the cost of liquid hydrogen storage and with more energy. Also, because the hydrogen particles are stored bound in the pores of the metal, they won’t explode when subjected to external shocks or heat. By solving these cost and reliability issues, hydrogen storage alloys have brought us one step closer to a future where hydrogen is used as an energy source. However, hydrogen storage alloys have their limitations, and researchers are working hard to find breakthroughs to address them. The main drawback of hydrogen storage alloys is their mass. Metals are among the densest substances. Since hydrogen storage alloys are also composed of a mixture of metals, they are dense and require a large mass to store the same amount of hydrogen compared to liquid hydrogen storage. As a result, hydrogen storage alloys have a high energy density, which is the amount of energy stored per volume, but a low specific energy, which is the amount of energy stored per mass. To compensate for this disadvantage of metals, researchers are actively investigating materials that fuse low-density organic materials with alloys. These materials are called MOFs. If it is possible to develop materials that retain the hydrogen storage properties of metals while reducing their mass through non-metallic elements, hydrogen energy technology will take a big leap forward.
The development of hydrogen storage alloys with high hydrogen energy density has brought us one step closer to advanced technologies that utilize hydrogen energy, such as long-distance space travel and electric vehicles, which were once thought to be a distant possibility. Hydrogen energy experts estimate that by 2030, 64% of light and passenger vehicles will be electric vehicles using hydrogen energy, and that hydrogen storage technology will be commercially available within 15 to 20 years. Hydrogen storage alloys are alloys made from a mixture of hydrogen-loving and non-hydrogen-loving metals that allow hydrogen to be stored in the metal or released out of the metal by controlling the strength of the interaction between each metal and hydrogen. Hydrogen storage alloys are broadly categorized into two types depending on how the interaction is controlled: those that use gaseous hydrogen as a raw material to control temperature and pressure, and those that use hydrogen in water molecules to control electrical potential. Although hydrogen storage alloys are heavy, if researches can solve this problem, it will be a breakthrough to store hydrogen in a high energy density and stable way. If hydrogen storage is actively researched and the limitations of hydrogen storage technology are overcome, we can expect the practical use of eco-friendly and highly efficient hydrogen energy in the near future.
